FDA Approves First At-Home Coronavirus Test; Likely Won’t Be Widely Available Until Spring
BY VANESSA ROMO
The first COVID-19 diagnostic at-home self-test that provides rapid results has been approved by the U.S. Food and Drug Administration, the agency announced Tuesday.
The Lucira COVID-19 All-In-One Test Kit is a molecular single-use test and is expected to cost $50 or less, the company said on its website.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn said in a statement.
Hahn added: “This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission.”
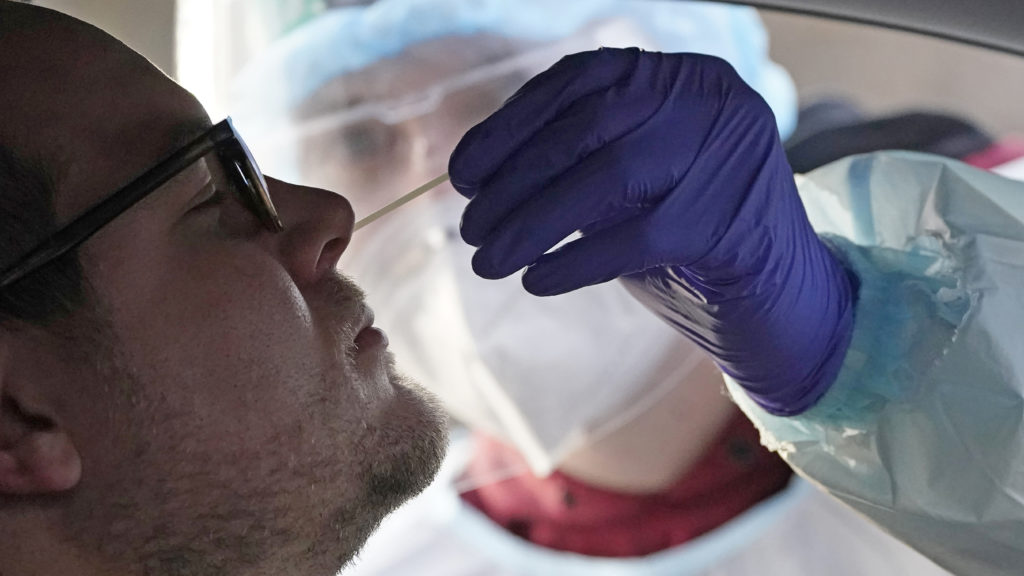
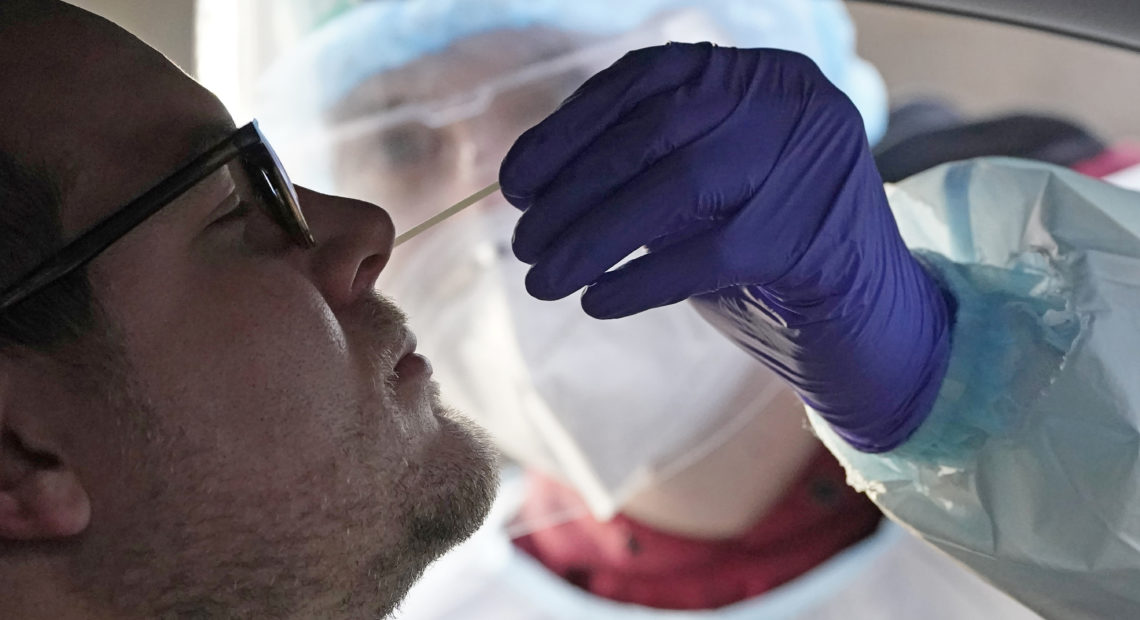
A coronavirus test is administered in Salt Lake City in Tuesday. At-home coronavirus tests will offer faster results than the traditional tests. CREDIT: Rick Bowmer/AP
The test works by swirling a self-collected swabbed sample in a vial, which is then placed in a hand-held test unit. It can provide results in 30 minutes or less, according to the agency. The unit’s light-up display shows whether a person is positive or negative for the SARS-CoV-2 virus.
Until now, people have had to visit a doctor’s office, clinic, hospital or some other site to have a sample taken or they could collect a sample at home for mailing to a lab for analysis.
The test will be available by prescription to people 14 and older who have symptoms that could indicate COVID-19. Patients under 14 could be given the test if it’s administered by a health care provider, the FDA said.
Initially, it will be available on a limited basis in Florida and California. It is expected to be available widely by the spring.
Health and Human Services Secretary Alex Azar said in a statement Tuesday that the new test adds “to our constantly expanding arsenal of COVID-19 testing options.”
“The Trump Administration has built the world’s largest testing system, and we will continue supporting both the development and manufacturing of cutting-edge options to make COVID-19 testing even easier and more accessible for the American people,” he said.
Jeff Shuren, the director of the FDA’s Center for Devices and Radiological Health, said that the test represents “a significant step toward the FDA’s nationwide response to COVID-19.”
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Shuren said.
Testing shortages have been a massive problem since the pandemic began, crippling the nation’s ability to fight the spread of the virus. The recent surge in cases has again put additional strain on the nation’s precarious coronavirus testing system, especially as more people try to get tested ahead of the holidays.
As NPR’s Rob Stein reported:
“Long lines are again forming in some places as the surge of infections drives a surge in demand for testing. Testing companies, lab directors and testing policy experts warn that waiting times for results could soon start to lengthen. In fact, one of the largest commercial testing companies Tuesday reported turnaround times had already started creeping up.”
The approval of at-home self-tests could have a big impact on the testing regime throughout the U.S.















